Molecular mass relative (Mr) calculated from the atomic mass number (Ar) of the elements in the molecule. Ar express the weight of the element relative to 1/12 atomic mass of C-12 isotope. Here is the example of the Mr NaCl calculation.
Since Ar Na=23 and Ar Cl=35,5
so, Mr NaCl is equal to the sum of that:
Mr NaCl = (1 x Ar Na) + (1 x Ar Cl) = (1 x 23) + (1 x 35,5) = 23 + 35,5 = 58,5
Now, you have to able to calculate the amount of Mr H2SO4 while known that Ar H = 1, Ar S = 32 and Ar O = 16. Right, the answer below.
Mr H2SO4 = (2 x Ar H) + (1 x Ar S) + (4 x Ar O) = (2 x 1) + (1 x 32) + (4 x Ar 16) = 2 + 32 + 64 = 98
The value of atomic mass number of some elements shown below.
H = 1 Li = 7 Mg = 12
C = 12 Na = 23 Ca = 40
O = 16 K = 39 Fe = 56
N = 14 Cl = 35,5 Cu = 63,5
Tuesday, 25 December 2012
Calculate the Molecule Mass Relative of the Compound
Label: anorganic chemistry, basic chemistry
Diposting oleh henryfekok di 12/25/2012 03:23:00 am 1 komentar
Saturday, 22 December 2012
Acid Overview
An acid simply defines as a substance in water solutions dissociate yielding hydrogens ions as only a positive ions. Some acids dissociate in one step yield one hydrogen ion called monobasic acid. Here is the example monobasic acid.
Others acid dissociate yield two or more hydrogen ions through two dissociation steps or more called polybasic acid.
Actually, in a aqueous solution, hydrogen ions doesn't exist. This proton combine with a water molecule form a hydronium ions by coordination covalent bond using a free pair electron from oxygen of the water molecule.
Label: anorganic chemistry, basic chemistry
Diposting oleh henryfekok di 12/22/2012 05:24:00 pm 5 komentar
Monday, 20 June 2011
Ionic Bonding
Label: anorganic chemistry, basic chemistry
Diposting oleh henryfekok di 6/20/2011 11:54:00 pm 3 komentar
The Stability of Elements
Label: basic chemistry
Diposting oleh henryfekok di 6/20/2011 11:48:00 pm 0 komentar
Flame Colour of Group IA (Group 1)
For example, a sodium ion in an unexcited state has the structure 1s2 2s2 2p6. When you heat it, the electrons gain energy and can jump into any of the empty orbitals at higher levels - for example, into the 7s or 6p or 4d or whatever, depending on how much energy a particular electron happens to absorb from the flame.
Because the electrons are now at a higher and more energetically unstable level, they tend to fall back down to where they were before - but not necessarily all in one go.
An electron which had been excited from the 2p level to an orbital in the 7 level, for example, might jump back to the 2p level in one go. That would release a certain amount of energy which would be seen as light of a particular color.
However, it might jump back in two (or more) stages. For example, first to the 5 level and then back to the 2 level.
Each of these jumps involves a specific amount of energy being released as light energy, and each corresponds to a particular color.
As a result of all these jumps, a spectrum of colored lines will be produced. The color you see will be a combination of all these individual colors.
The exact sizes of the possible jumps in energy terms vary from one metal ion to another. That means that each different ion will have a different pattern of spectral lines, and so a different flame color.
The colors
The colors in the table are just a guide. Almost everybody sees and describes colors differently. I have, for example, used the word "red" several times to describe colors which can be quite different from each other. Other people use words like "carmine" or "crimson" or "scarlet", but not everyone knows the differences between these words - particularly if their
Li red
Na strong persistent orange
K lilac (pink)
Rb red (reddish-violet)
Cs blue? violet? (see below)
What do you do if you have a red flame colour for an unknown compound and don't know which of the various reds it is?
Get samples of known lithium, strontium (etc) compounds and repeat the flame test, comparing the colours produced by one of the known compounds and the unknown compound side by side until you have a good match.
________________________________________
Note: I don't have confidence in the caesium flame colour. There is total disagreement about this on the web and in the books I have looked at, and I have never seen this flame colour myself. However, I have received a helpful email from a student who says: "At my school we did some flame testing experiments, and . . . caesium is actually either blue or violet, depending on the way you look at it. I think it looks more violet than blue, but it sort of changes each time you do it." (Kara Gates, March 2006). If you thought chemistry was clear-cut, you are sadly mistaken!
________________________________________
Label: analytic chemistry, anorganic chemistry, basic chemistry
Diposting oleh henryfekok di 6/20/2011 11:38:00 pm 0 komentar
Monday, 7 September 2009
Ionization Energy
Ionization energy is the energy required by an atom in the form of gas to remove one electron to form positive ions.
 The lower the ionization energy, the easier an electron released an atom to form negative ions.
The lower the ionization energy, the easier an electron released an atom to form negative ions.
 In the periodic table, ionization energy values showed periodicity. In a period from left to right, the ionization energy increased. In one class, from left to right, ionization energy decreased.
In the periodic table, ionization energy values showed periodicity. In a period from left to right, the ionization energy increased. In one class, from left to right, ionization energy decreased.

Label: anorganic chemistry, basic chemistry
Diposting oleh henryfekok di 9/07/2009 05:18:00 am 9 komentar
Friday, 4 September 2009
Electron Affinity
Electron affinity is the energy released by an atom (in the form of gas) when an electron capture to form negative ions. Since the energy is released, given the price of the electron affinity of the minus sign.
 The greater the energy released, the negative ions are formed more stable. IIA group elements and VIIIA not form a stable negative ions. Prices positive electron affinity.
The greater the energy released, the negative ions are formed more stable. IIA group elements and VIIIA not form a stable negative ions. Prices positive electron affinity.
In one period, from left to right, electron affinity values tend to increase. In one class, from top to bottom, the electron affinity values tend to decrease.

Label: anorganic chemistry, basic chemistry
Diposting oleh henryfekok di 9/04/2009 05:26:00 am 1 komentar
Wednesday, 10 June 2009
Introduction of Chemical Bonds
An atom consists of the atomic core and the atom's skins. Atomic core consists of proton particles that is positive particles and neutrons that ia neutral particles. Meanwhile, electron skins contains electron that is negative particles. The place where electrons found is called the orbital. Electrons first put themselves on the orbitals which has the lowest energy level.
Two or more atoms can form a chemical bond using the valence electrons in order to form a molecule. If the atoms does not have a strong difference electronegativity (or little difference of electronegativity), valences electron of atoms are used together formed a covalent bond. If the atoms has a large difference of electronegativity, the atoms are formed ion bonds.
The atoms bonded to obtain stable configuration that is formed as electrons configuration of noble gas elements.
In the covalent bond, electrons used together by the atoms so that the atoms has electron configuration such as electron configuration noble gas elements. For example, hydrogen has 1 valence electron and oxygen has 6 valence electrons. Both types of elements are formed of water molecules (H2O).
 If a bond has a pair of electrons used to bond, called single covalent bond. There are also covalent bong type with two pairs of electrons, called double covalent bond. If the covalent bond using three electron pairs, called the triple cobalent bond. In some cases, the electron pair shared come from only one atom of it, called coordination covalent bond or coordinates covalent bond or covalent dativ bond or semipolar covalent bond.
If a bond has a pair of electrons used to bond, called single covalent bond. There are also covalent bong type with two pairs of electrons, called double covalent bond. If the covalent bond using three electron pairs, called the triple cobalent bond. In some cases, the electron pair shared come from only one atom of it, called coordination covalent bond or coordinates covalent bond or covalent dativ bond or semipolar covalent bond.
At the ion bond, the atoms has a high value of electronegativity capture elctrons form a negative ion, while the atoms that has a low value of electronegaivity release the valense electrons formed positive ion. With the capture or release electrons, the atoms become stable formed electron configuration like elements configuration of noble gas. Although the atoms in the compound does not use the pair electron together, but the atoms have a strong bond each other because the atoms cargo is different. In an ion compound, all the ions form a crystal lattice structure.
Label: anorganic chemistry, basic chemistry
Diposting oleh henryfekok di 6/10/2009 10:20:00 am 0 komentar
Friday, 10 April 2009
Measuring pH Solution Using Acid Base Indicator
| Measurement Tool | pH range | Acid | Base |
| Litmus Phenolphtalein Metil orange Metil red Bromtimol blue Universal indicator pH meter | 5.5 - 8 8.3 - 10 2.8 - 4 4.2 - 6.3 6.0 - 7.6 0 - 14 0 - 14 | Red No color Red Red Yellow -- -- | Blue Red Yellow Yellow Blue -- -- |
Example: Next stretch indicator data basa acid.
| Indicator | Stretch pH | Changes color |
| Metil purple Bromkesol green Bromtimol blue | 0.5 - 1.5 3.8 - 5.4 6.0 - 7.6 | Yellow - purple Yellow - blue Yellow - blue |
given:
with metil purple indicator, purple
with bromkesol green indicator, blue
with bromtimol blue indicator, yellow
Determine the pH range of the solution!
Answer:
According to data range pH indicator:
With metil purple indicator, purple, the pH solution > 1.5.
With bromkesol green indicator, blue, the pH solution > 5.4.
With bromtimol blue indicator, yellow, the pH solution <>
Label: basic chemistry
Diposting oleh henryfekok di 4/10/2009 12:31:00 am 1 komentar
Thursday, 2 April 2009
Metal and Non-metallic Elements
Nowadays, more than 100 elements have been found. Elements have certain properties that distinguish between the one elements with the other elements. We can distinguish the elements to the metal elements, metaloid elements and non-metallic elements.
Some of the properties of the metal:
Malleability: the metal can be formed without cracks.
Ductility: the metal can be drawn without a change in the surface.
Toughness: metal can be crooked.
Hardness: resistance to the penetration of metal or other hard objects puncture.
Commonly non-metal elements in gas phase at room temperature. While metaloid elements have the properties between metal elements and non-metallic elements.
In the table of periodic system of the elements, non-metallic elements located on the left side (except hydrogen) and non-metallic elements is located on the right side. Metal and non-metallic elements is separated by the metaloid elements which is located in the middle table of periodic system of the elements.
Metaloid elements are Boron (B), Silicon (Si), Germanium (Ge), Arsenic (As), Antimony (Sb), Tellurium (Te) Polonium (Po), Astatine (At). Some of the metal elements are Sodium (Na), Iron (Fe), Coppre (Cu) etc. Some of the non-metallic elements are hydrogen (H), Helium(He), Oxygen (O), Nitrogen (N) etc.
Label: anorganic chemistry, basic chemistry
Diposting oleh henryfekok di 4/02/2009 05:35:00 pm 0 komentar
Friday, 27 March 2009
Water Equilibrium Constant and Acid Base Strength
Water Equilibrium Constant
Water is a very weak electrolyte. Ionization occurs in water:
H2O → H+ + OH-
K [H2O] = [H+] [OH-]
Kw = [H+] [OH-]
At a temperature of 25ºC, Kw = 1.10-14, so that [H+] = [OH-] = 1.10-7.
Acid solution [H+]> 1.10-7.
Base solution [OH-] < 1.10-7.
Acid Base Strength
Acid base strength expressed in H+ or OH- concentration. Acid with the high concentration of H+ has greater acid strength than with the acid H+ with low concentration. Similarly base with its concentration of OH-.
Example:
HCl → ....H+ ....+ ...Cl-
0.1 M .. 0.1 M 0....0.1 M
H2SO4 → ....2H+ ...+ ..SO42-
0.1 M .....0.2 M...... 0.1 M
At the same concentration (0.1 M), H2SO4 is more acid than HCl because the H2SO4 has greater H+ concentration than HCl.
Label: basic chemistry
Diposting oleh henryfekok di 3/27/2009 10:40:00 pm 0 komentar
Acid Base Theory
1. Arrhenius Theory
Acid: electrolyte substance which in water produce H+ ion.
Example: HBr(aq) → H+(aq) + Br-(aq).
Base: electrolyte substance which in water produces OH-.
Example: KOH(aq) → K+(aq) + OH-(aq).
2. Bronsted Lowry Theory
Acid: substance that gives proton or H+.
Base: oxygen proton or accept a H+.
Example: H2O + NH3 → NH4+ + OH-
acid1 basa1 acid2 base2
Base acid conjugation couple has characteristics deviate only 1 H+.
For the reaction on the spouse: H2O and OH-, NH3 and NH4+.
Substance amphipirotic/amfoter: substances that can give or receive
the proton (H+). Ex: H2O, H2PO4-,HPO42-.
3. Lewis Theory
Acid: electron pair acceptor.
Base: electron pair donor.
Example: BF3 + :NH3 → H3N:BF3
NH3 to give a pair of electrons to BF3, NH3 as base and BF3 as acid.
Label: basic chemistry
Diposting oleh henryfekok di 3/27/2009 09:40:00 pm 0 komentar
Friday, 20 March 2009
Penggolongan Materi (Material Classification)
Ilmu kimia mempelajari materi. materi adalah sesuatu yang mempunyai massa dan menempati ruang. Materi dapat digolongkan ke dalam zat tunggal dan campuran.
ZAT TUNGGAL
- Unsur
- Senyawa
Unsur merupakan zat tunggal yang tidak bisa dipisahkan menjadi zat lain yang lebih sederhana. Contoh: hidrogen (H), oksigen (O), besi (Fe) dan lain-lain.
Senyawa adalah zat tunggal yang dapat dipisahkan menjadi zat lain yang lebih sederhana. Suatu senyawa hanya dapat dipisahkan menjadi unsur-unsur penyusunnya melalui perubahan kimia. Contoh senyawa: air (H2O), alkohol (C2H5OH) dan lain-lain.
- Larutan
- Koloid
- Suspensi
Larutan adalah campuran homogen yang terdiri dari dua jenis zat atau lebih. Homogen artinya tidak ada bidang batas antara zat yang satu dengan yang lain (meski diamati menggunakan mikroskop ultra) atau mempunyai satu fasa. Larutan sering disebut secara spesifik sebagai larutan sejati. Ukuran partikel larutan sejati kurang dari 1.10-9 nm. Contoh: larutan gula.
 Koloid merupakan jenis campuran yang memiliki karakteristik khusus sehingga dipelajari pada pembahasan tersendiri. Secara kasat mata koloid terlihat homogen, tetapi jika diamati menggunakan mikroskop ultra akan terlihat jelas bahwa koloid merupakan campuran heterogen. Ukuran partikel koloid antara 1.10-9 - 1.10-7 nm. Contoh: susu, agar-agar dan kabut.
Koloid merupakan jenis campuran yang memiliki karakteristik khusus sehingga dipelajari pada pembahasan tersendiri. Secara kasat mata koloid terlihat homogen, tetapi jika diamati menggunakan mikroskop ultra akan terlihat jelas bahwa koloid merupakan campuran heterogen. Ukuran partikel koloid antara 1.10-9 - 1.10-7 nm. Contoh: susu, agar-agar dan kabut.
(gambar diambil dari: http://roseskitchen.files.wordpress.com/2007/06/pandan-custard-agar-agar-slices.jpg)
Suspensi merupakan campuran heterogen dengan ukuran partikel lebih besar dari 1.10-7 nm. Contoh: campuran tepung terigu dengan air.
Chemistry learning all about materials. The material is anything that has mass and occupies space. Materials can be classified into a single substance and mixture.
SINGLE SUBSTANCE
1. Element
Element is a single substance that can not be separated into other simple substances. Example: hydrogen (H), oxygen (O), iron (Fe) and others.
2. Compound
Compound is a single substance that can be separated into other simple substances. A compound can only be separated into elements by chemical changes. Example compound: water (H2O), alcohol (C2H5OH) and others.
MIXED
1. Solution
Solution is homogeneous mixture and consists of two or more kinds of substances. It means that is no homogeneous field boundary between the substance of one another (although observed using ultra-microscope) or to have one phase. Solution is often referred to specifically as the pure solvent. The size of pure solvent particles less than 1.10-9 nm. Example: sugar solution.

2. Colloid
Colloid is a mixture that have special characteristics so that discussed in aother part. The colloidal look homogeneous, but when colloid observed using a ultra-microscope will be visible to clear that the colloid is a heterogeneous mixture. The size of colloidal particles between 1.10-9 - 1.10-7 nm. Examples: milk, jelly and fog.
(picture taken from: http://www.jaylichtman.com/pictures/picture-oregon-city-or-bridge-in-fog.JPG)
3. Suspensi
Suspensi is a heterogeneous mixture of particles with a size greater than the 1.10-7 nm. Example: a mixture of wheat flour with water.
Label: basic chemistry
Diposting oleh henryfekok di 3/20/2009 07:14:00 am 0 komentar
Thursday, 19 March 2009
Perubahan Kimia dan Perubahan Fisika
 Perubahan fisika tidak ditandai dengan terbentuknya zat baru. Sedangkan perubahan kimia ditandai dengan terbentuknya zat baru.
Perubahan fisika tidak ditandai dengan terbentuknya zat baru. Sedangkan perubahan kimia ditandai dengan terbentuknya zat baru.
Perubahan fisika lebih kita kenal sebagai perubahan wujud zat. Pada perubahan wujud zat, tidak ada zat baru yang terbentuk. Zat tersebut meski telah berubah wujudnya tetapi masih mempunyai rumus molekul kimia yang sama. Macam-macam perubahan fisika:
- Mencair: perubahan wujud zat dari padat ke cair.
- Menguap: perubahan wujud zat dari cair ke gas.
- Menyublim: perubahan wujud zat dari gas ke padat/sebaliknya.
- Mengembun: perubahan wujud zat dari gas ke cair.
- Membeku: perubahan wujud zat dari cair ke padat.
Perubahan kimia kita kenal sebagai reaksi kimia ditandai dengan beberapa ciri:
- perubahan warna.
- perubahan suhu.
- timbulnya endapan.
- timbulnya gas.
C8H8(g) + 12 O2(g) ----> 8 CO2(g) + 4 H2O(g)
bensin + oksigen ----> karbondioksida + uap air
Terlihat bahwa terbentuk zat baru yaitu karbondioksida dan uap air yang memiliki rumus molekul berbeda dengan zat awal yaitu bensin dan oksigen.
============================================================
Physical change is not marked with the new substance. While the chemical changes are marked with the new substance.
Physical change as we know called as phase substance. There is no new substance is created. Although these substance have phase changed but these substance still have the same chemical molecule formula. Various physical changes:
1. Melt: change in form of solid matter to liquid.
2. Evaporate: change in form of liquid matter to gas.
3. Sublimation: change in form of solid matter to gas and vice versa.
4. Dew: change in form of gas matter to liquid.
5. Frozen: change in form of liquid matter to solid.
Changes in ice melt into water is an example of physical changes. Ice and water have the same molecular formula, which is H2O, so that no new substance is created.
Chemical changes we know as a chemical reaction by a few characteristics:
1. changes of color.
2. changes in temperature.
3. the occurrence of precipitation.
4. the occurrence of gas.
In the combustion of fuel gas there is chemical process change occurs. If we consider gas consists of octane, burning gas reaction:
C8H8 (g) + 12 O2 (g) ----> 8 CO2 (g) + 4 H2O (g)
fuel + oxygen ----> carbon dioxide + water vapor
That form new substances, namely carbon dioxide and water vapor that has a different molecular formula with the initial substances, octane and oxygen gas.
Label: basic chemistry
Diposting oleh henryfekok di 3/19/2009 04:45:00 pm 0 komentar
Sunday, 15 March 2009
Tentang Atom dan Unsur (About Atoms And Elements)
Partikel terkecil dari suatu materi disebut atom. Jika kita punya sepotong logam aluminium lalu membaginya menjadi bagian-bagian sangat kecil sehingga tidak dapat dibagi lagi, maka bagian terkecil itu disebut atom.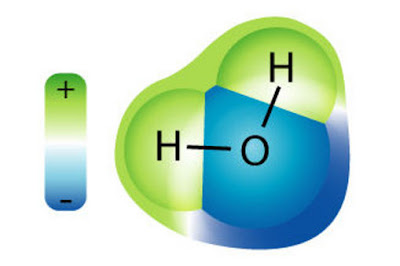
Unsur adalah jenis/macam atom. Dalam sepotong logam aluminium terdapat banyak unsur aluminium yang bersatu melalui ikatan logam. Dalam air (H2O) terdapat dua jenis atom, yaitu unsur hidrogen dan oksigen. Jumlah unsur hidrogen = 2 dan jumlah unsur oksigen = 1.
(gambar diambil dari http://www.sciencelearn.org.nz/var/sciencelearn/storage/images/contexts/icy_ecosystems/sci_media/water_molecule/21463-1-eng-NZ/water_molecule_full_size_landscape.jpg)
Kimia menggunakan simbol/lambang untuk mewakili unsur. Simbol/lambang merupakan gambar atau huruf yang digunakan untuk mewakili sesuatu. Kimia menggunakan satu atau dua huruf untuk mewakili unsur. Simbol untuk aluminium adalah Al. Simbol untuk oksigen adalah O dan lain-lain.
Kesimpulannya:
O merupakan unsur karena melambangkan jenis atom oksigen (unsur oksigen).
Atom-atom dapat bergabung membentuk molekul. Ada molekul unsur dan molekul senyawa.
Molekul unsur hanya terdiri dari satu jenis unsur, contoh H2, O2, N2.
Molekul senyawa terdiri dari dua jenis unsur atau lebih, contoh H2O, NaCl.
Kesimpulannya :
O2 merupakan molekul unsur, terdiri dari 2 buah atom, jenis atom/unsurnya adalah oksigen.
====================================================================================
The smallest particles of a material called atom. If we have a metal piece aluminum then divide it to the very small parts so it can not be divided again, the smallest part is called atom.
Element is the type of / kind of atom. In aluminium metal piece there are many elements that bond together through the metal. In water (H2O) There are two types of atom, the element hydrogen and oxygen. Number of hydrogen element = 2 and Number of oxygen element = 1.
Chemist using symbols to represent the elements. Symbol is a letter that is used to represent something. Chemical use one or two letters to represent the elements. The symbol for aluminum is Al. The symbol for oxygen is O, and others.
In conclusion:
O is an element because it represents the type of oxygen atom (oxygen elements).
Atom-atom which formed a molecule can join. There are molecular element and molecular compound.
Molecular element consists of only one type of element, for example H2, O2, N2.
Molecular compound consisting of two or more types of elements, eg H2O, NaCl.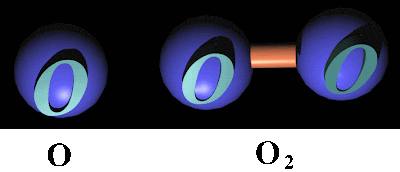
In conclusion:
O2 is a molecular element, consist of 2 pieces atom, the type of atom (element) is oxygen.
(picture taken from: http://www.monroecc.edu/depts/pstc/backup/o22.gif)
Label: basic chemistry
Diposting oleh henryfekok di 3/15/2009 03:23:00 pm 0 komentar







